The unabated high cost of prescription medicines in America has prompted a public outcry for solutions from state and federal policymakers as well as health care leaders. Meanwhile, FDA-approved generic medicines continue to generate competition for more expensive brand drugs and reduce costs for America’s patients for 35 years running. Generic drugs are a core component to lowering drug spending, and the use of generic medicines saved $313 billion in 2019 alone. This means greater affordability and access to care for millions of patients, as well as lower spending for government and private sector payers. As voters cite drug costs as one of their top issues for the 2020 campaign, it is critically important to ensure generic drugs are accessible to patients.
New generic competitors are particularly important as brand drug prices increase, sometimes reaching $1 million or more. First generics – those drugs approved by the Food and Drug Administration (FDA) as the first competitor to a brand – benefit patients through new competition and lower prices. But although the FDA has been approving generic drug applications at a record-setting pace, access to these savings – particularly from first generics – continues to be under threat.
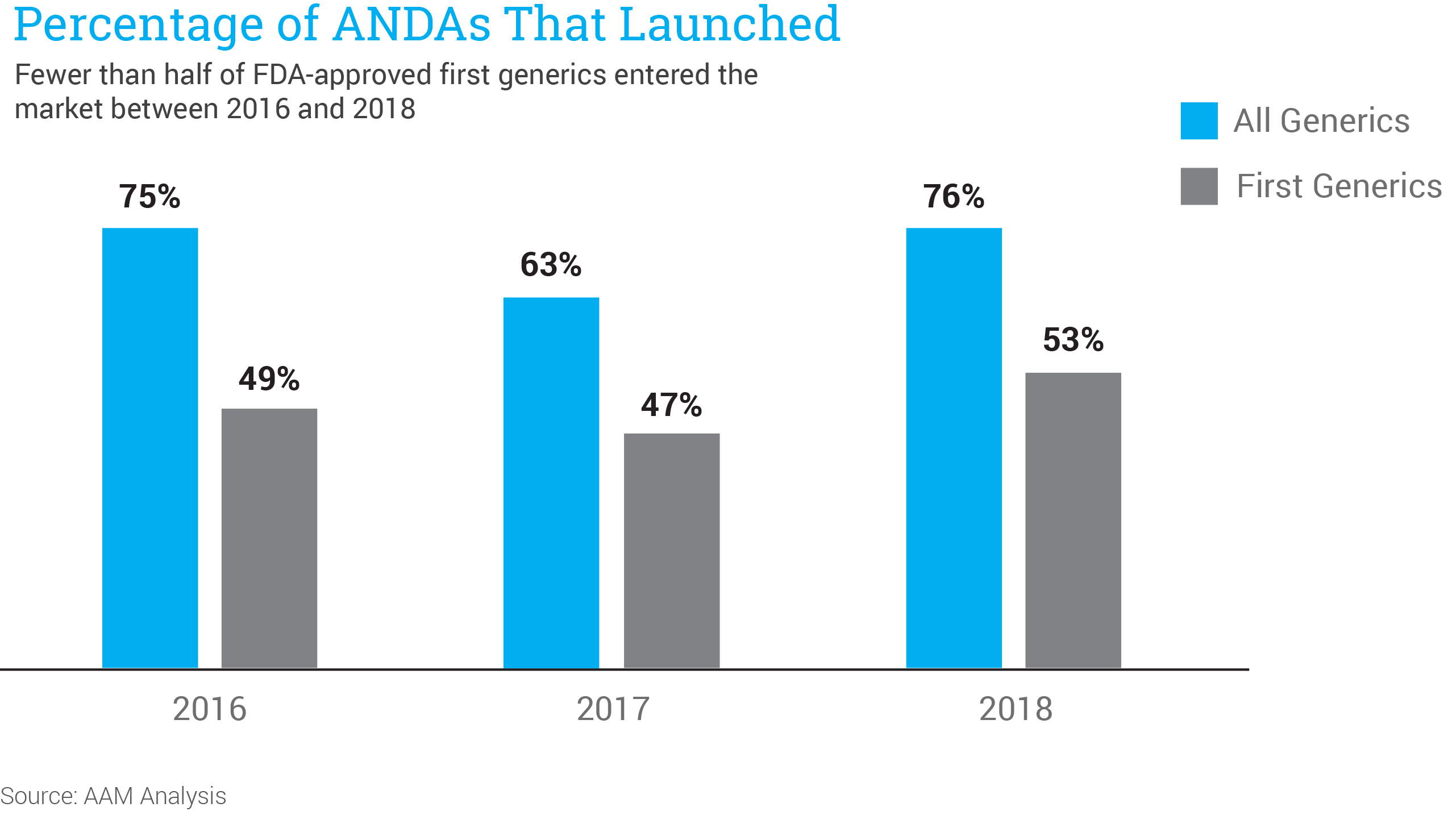
Data developed for and featured in this white paper highlight the challenges facing new generic competition, revealing that fewer than half of the first generics approved by FDA since 2016 are commercially available to patients.
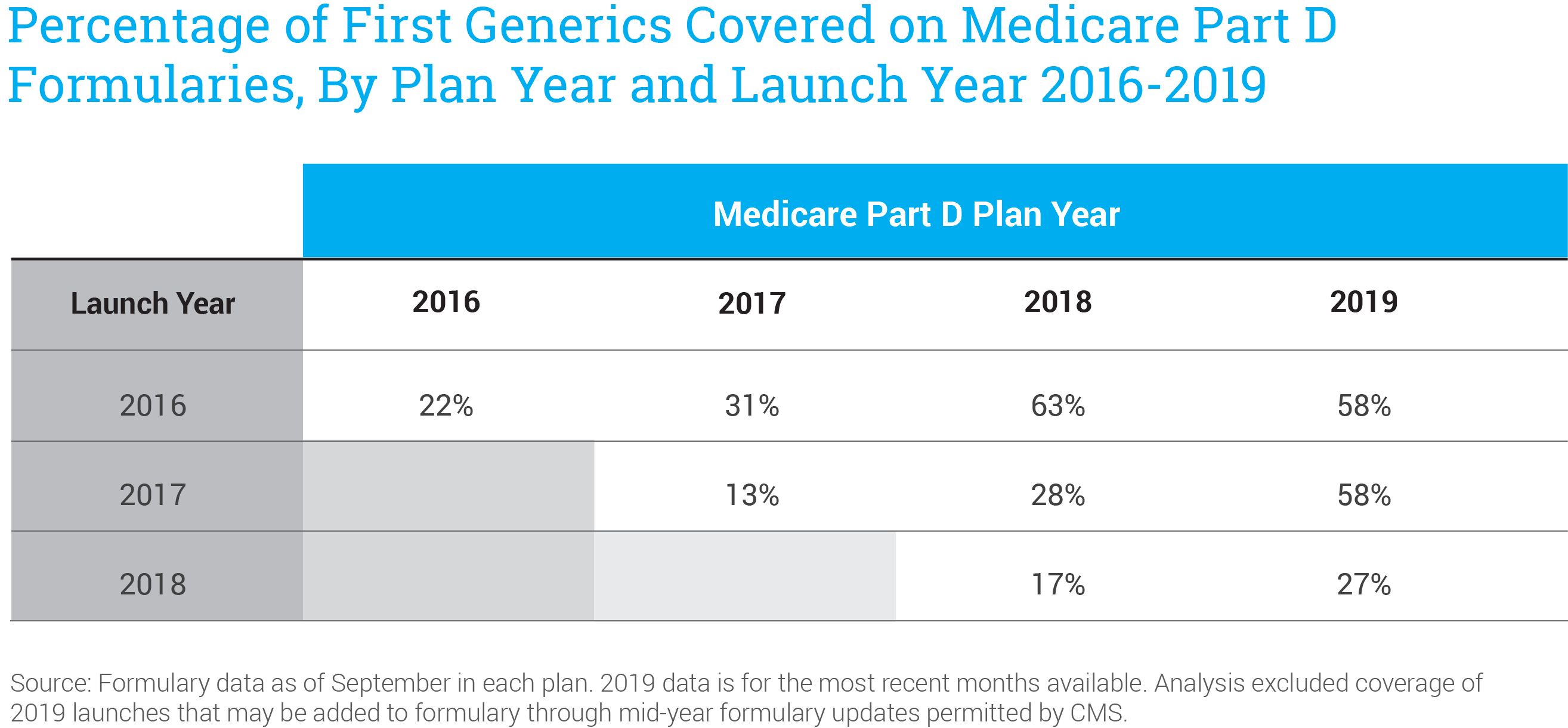
Moreover, of those that are commercially available, AAM found that only about half were included on formularies of Medicare Part D plans. Once they are added to formularies, first generics are routinely placed on expensive brand drug tiers with higher patient copays, rather than on generic tiers with lower cost-sharing. As a result, seniors do not benefit from lower prices and lower out-of-pocket costs, and taxpayers continue to pay for high-priced brand drugs.
This is a result of two design features of the Medicare Part D program, including the availability of brand drug rebates on high-priced brand drugs. Policymakers can solve this issue by updating the Part D program to ensure that first generics are covered at launch with lower cost-sharing, including through a dedicated tier for specialty generic and biosimilar medicines. These changes could save seniors more than $4 billion yearly and save money for taxpayers.
Sick of paying outrageous prices for your prescription medicines? Send a message to your lawmakers to tell them, “This Doesn’t Add Up.”



