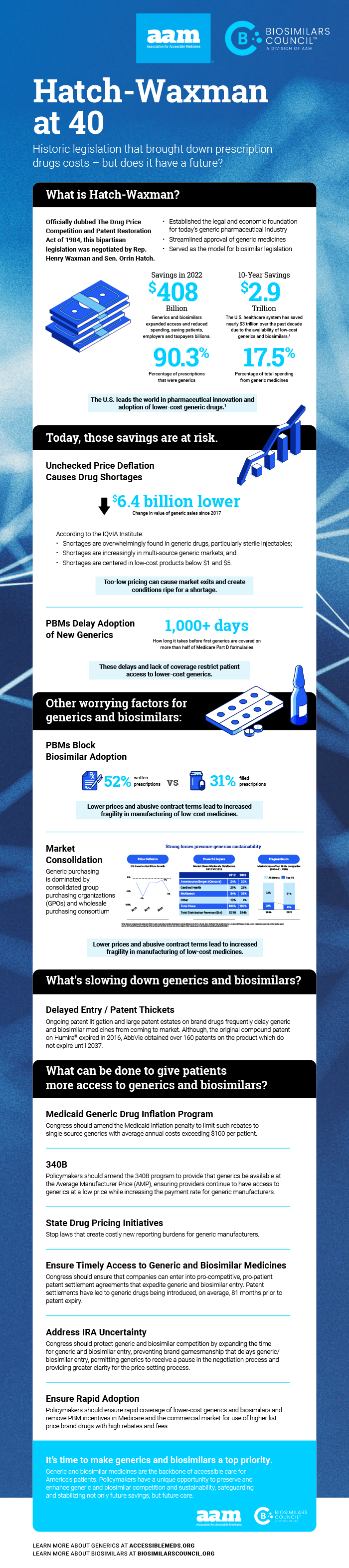
Future patient access and savings at risk without action
WASHINGTON (September 24, 2024) — Today marks the 40th Anniversary of the enactment of Hatch-Waxman, the landmark Drug Price Competition and Patent Restoration Act of 1984. The Association for Accessible Medicines and its Biosimilars Council today marked the anniversary.
40 years ago today, the generic medicines industry was empowered by Hatch-Waxman which ushered in trillions in savings for patients and the entire heath care ecosystem,
said David Gaugh, Interim President and CEO of AAM. The competition provided by this law was a success as over 90% of all prescriptions today are generics or biosimilars yet only 13 percent of total drug spending. But over the years, Hatch-Waxman is being chipped away and now more than ever we need action from our regulators and legislators to protect patient access to lower-cost drugs for the next 40 years and beyond.
AAM earlier released a white paper titled, Hatch-Waxman Turns 40. Is it Over the Hill? (Or is the Hill over Hatch-Waxman…) outlining the lasting benefits the generic drug industry has provided to patients, employers and taxpayers and detailing the unprecedented challenges that call into question the viability of sustainable competition from low-cost medicines in the decades to come. AAM also released the infographic below.
Hatch-Waxman has saved trillions of dollars but is now sorely in need of updates to keep up with the ever changing landscape, especially with respect to the incredible potential of biosimilars, which did not exist 40 years ago,
said Craig Burton, Executive Director of the Biosimilars Council. Patent thickets, attacks on skinny labeling, PBM pricing schemes, misuse of generic tiers for Medicare patients are just a few of the many issues facing our industry and getting in the way of lower-cost medicines reaching patients.
The results of Hatch-Waxman have provided unprecedented savings according to AAM’s most recent annual savings report, in the last ten years alone, the use of generic and biosimilar drugs has saved patients and the U.S. healthcare system over $3 trillion dollars. But this past success and increase in patient access to care is currently at risk.
For media inquiries, contact the Communications department at media@accessiblemeds.org.
About AAM
The Association for Accessible Medicines, your generics and biosimilars industry, is driven by the belief that access to safe, quality, effective medicine has a tremendous impact on a person’s life and the world around them. Generic and biosimilar medicines improve people’s lives, improving society and the economy in turn. AAM represents the manufacturers of finished generic pharmaceuticals and biosimilars, manufacturers of bulk pharmaceutical chemicals, and suppliers of other goods and services to the generic industry. Generic pharmaceuticals are 90 percent of prescriptions dispensed in the U.S. but only 13.1 percent of total drug spending.
About the Biosimilars Council
The Biosimilars Council, a division of the Association for Accessible Medicines (AAM), works to ensure a positive environment for patient access to biosimilar medicines. The Biosimilars Council is a leading source for information about the safety and efficacy of more affordable alternatives to costly brand biologic medicines. Areas of focus include public and health expert education, strategic partnerships, government affairs, legal affairs and regulatory policy. More information is available at www.biosimilarscouncil.org.