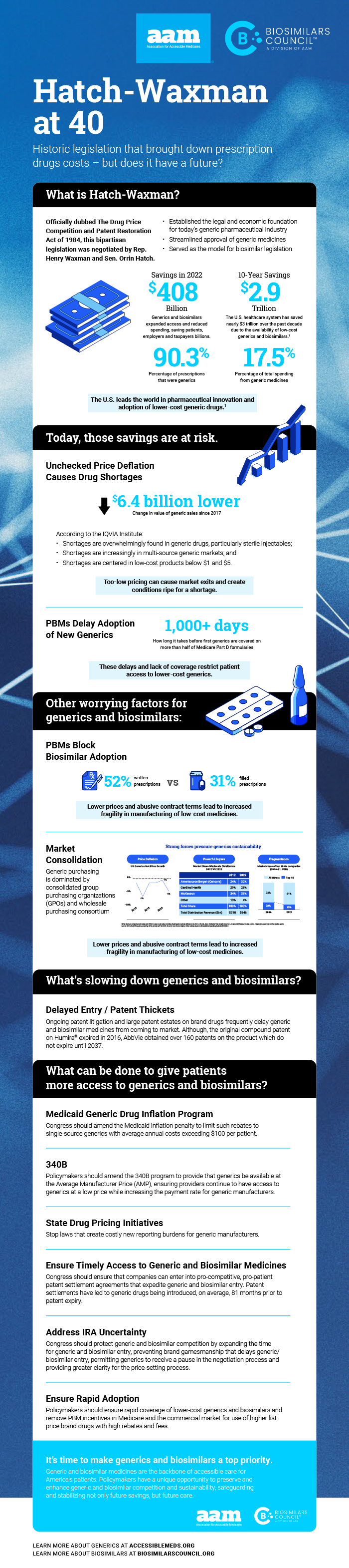
The 1984 law has enabled trillions of dollars in savings; but the industry is at risk
TAMPA, FL (February 6, 2024) — The Association for Accessible Medicines, the leading trade association for generic and biosimilar manufacturers, today released a new report touting the lasting benefits the generic drug industry has provided to patients, employers and taxpayers in the forty years since the enactment of Hatch-Waxman, the landmark Drug Price Competition and Patent Restoration Act of 1984, but also the unprecedented challenges facing the industry calling into question the viability of sustainable competition from low-cost medicines.
2024 marks the 40th anniversary of Hatch-Waxman, which created a pathway for the introduction of lower-cost generic drugs. The results have yielded unparalleled savings successes over the last several decades: according to AAM’s most recent annual savings report, in the last ten years alone, the use of generic drugs has saved patients and the U.S. healthcare system almost $3 trillion dollars. But this track record of success—and the resulting increase in patient access to care—is currently at risk.
Since the introduction of this law, we have all seen incredible savings,
said David Gaugh, AAM’s Interim President & CEO. But even as we celebrate the success of forty years of Hatch-Waxman, those savings, the increased patient access, and a model of generic adoption that leads the world, are at risk. It is incumbent upon us as leaders of this industry to come together with policymakers and other key stakeholders to safeguard and stabilize not only future savings, but future care to America’s patients.
The new report, Hatch-Waxman at 40: Is it over the hill (or is The Hill over Hatch-Waxman?),
details the unprecedented challenges facing the generic and biosimilar industries today, including unchecked price deflation, fewer new generics and slower market penetration for new generics, slower than expected biosimilar adoption, and drug shortages. The report seeks to educate policymakers on the threats to future generic and biosimilar savings, the root causes generating these threats, and suggests legislative and regulatory action that can be taken by policymakers in Congress, the White House and state governments to preserve and enhance generic and biosimilar competition and sustainability.
The report was released as generic and biosimilar industry leaders, key stakeholders and policymakers gathered in Tampa for AAM’s annual conference, Access! 2024, to consider the business, breakthroughs, and politics that shape the industry—and in particular, the economic risk to its long-term sustainability.
The report and accompanying infographics can be found here:
For media inquiries, contact the Communications department at media@accessiblemeds.org.
About AAM
AAM is driven by the belief that access to safe, quality, effective medicine has a tremendous impact on a person’s life and the world around them. Generic and biosimilar medicines improve people’s lives, improving society and the economy in turn. AAM represents the manufacturers and distributors of finished generic pharmaceuticals and biosimilars, manufacturers and distributors of bulk pharmaceutical chemicals, and suppliers of other goods and services to the generic industry. Generic pharmaceuticals are 90 percent of prescriptions dispensed in the U.S. but only 17.5 percent of total drug spending.
About the Biosimilars Council
The Biosimilars Council, a division of the Association for Accessible Medicines (AAM), works to ensure a positive environment for patient access to biosimilar medicines. The Biosimilars Council is a leading source for information about the safety and efficacy of more affordable alternatives to costly brand biologic medicines. Areas of focus include public and health expert education, strategic partnerships, government affairs, legal affairs and regulatory policy. More information is available at www.biosimilarscouncil.org.