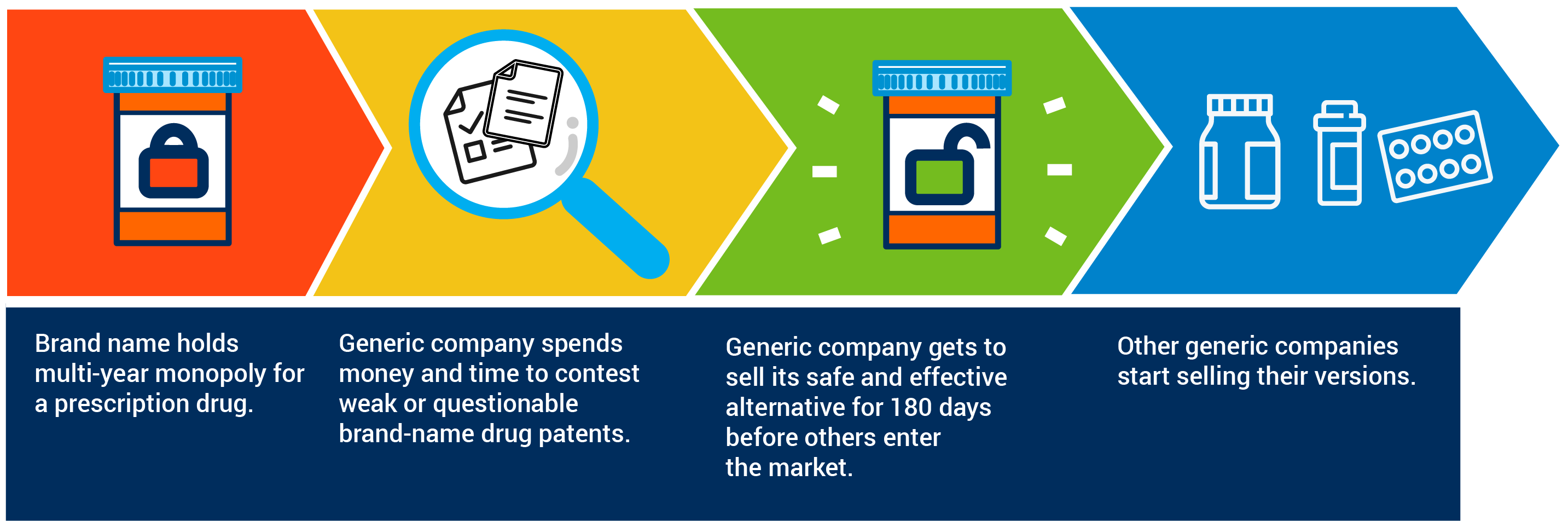
Decades ago, Congress created a sensible system to encourage developers of more affordable generic medicines to challenge patents protecting expensive brand-name drugs. These brand-name drug patents keep a more affordable, safe and effective generic option off the market and out of the hands of patients.
The system that Congress created 35 years ago is based on the idea that for generic companies to incur the tremendous financial and legal costs of contesting brand-name drug patents they would need some kind of reward or incentive. Congress wisely settled on providing the first generic company that challenges a brand-name drug patent a period of time to sell its competing medicine before other generic companies start offering their own alternatives.
By promoting patent challenges, a period of exclusivity encourages earlier entry of safe and effective generic alternatives that are less expensive than the brand. These 180 days have been critical to ensuring that American patients can rely on the ready availability of safe, effective and more affordable generic medicines.
But this track record of success is at risk. Congress is considering a significant change that, if enacted, would put patient access to more affordable generic medicines in jeopardy. If you are concerned about the rising cost of prescription drugs, now is the time to contact your representatives in the House and Senate to urge them to oppose inclusion of the BLOCKING Act (H.R. 938) in any legislation acted on before the end of the year.
Read Blog by AAM CEO Dan Leonard
A January 2020 report from Matrix Global Advisors, “The Unintended Economics of the BLOCKING Act,” finds the delay of just one generic medicine will increase prescription drug costs for patients by $1.7 billion.