Congress is currently working on bipartisan proposals to bring down the high cost of prescription drugs, a goal we can all agree on. However, no good deed goes unpunished. Section 205 of the Lower Health Care Costs Act of 2019 would severely punish patients and consumers – the very people Congress is trying to help.
Generic drugs have been a resounding success for U.S. patients and the health care system, cutting the high cost of brand-name prescription drug and increasing access to life-saving medicines. But Section 205, currently being considered by the Senate HELP Committee, would undermine incentives for generic manufacturers to challenge brand-name patent monopolies. And without a competitive marketplace, prices for brand-name medicines will continue to skyrocket.
By all accounts, the growth of the generic drug industry has been a boon to the health and pocketbooks of all Americans. Currently, 90 percent of all prescriptions dispensed in the U.S. are for generic drugs, and patients have saved $2 trillion over the last decade, including $293 billion in 2018, as a result of the availability of low-cost generics. Moreover, because generic competition lowers prices, more Americans than ever now have access to treatments that previously would have been unaffordable.
The key to this success has been ensuring that generic companies have adequate incentives to challenge brand-name drug monopolies. The current law offers generic companies a “brass ring”: If they are the first to take the significant risk and expense of challenging patents that protect the brand-name drug beyond the initial new drug innovation, they are rewarded with a short window during which they are the only generic product on the market. This “180-day exclusivity” period results in vigorous efforts by generic companies to challenge or innovate around weak patents, thereby opening a pathway that other generic companies can follow. This, in turn, creates earlier availability of affordable generic medicines and more generic competition.
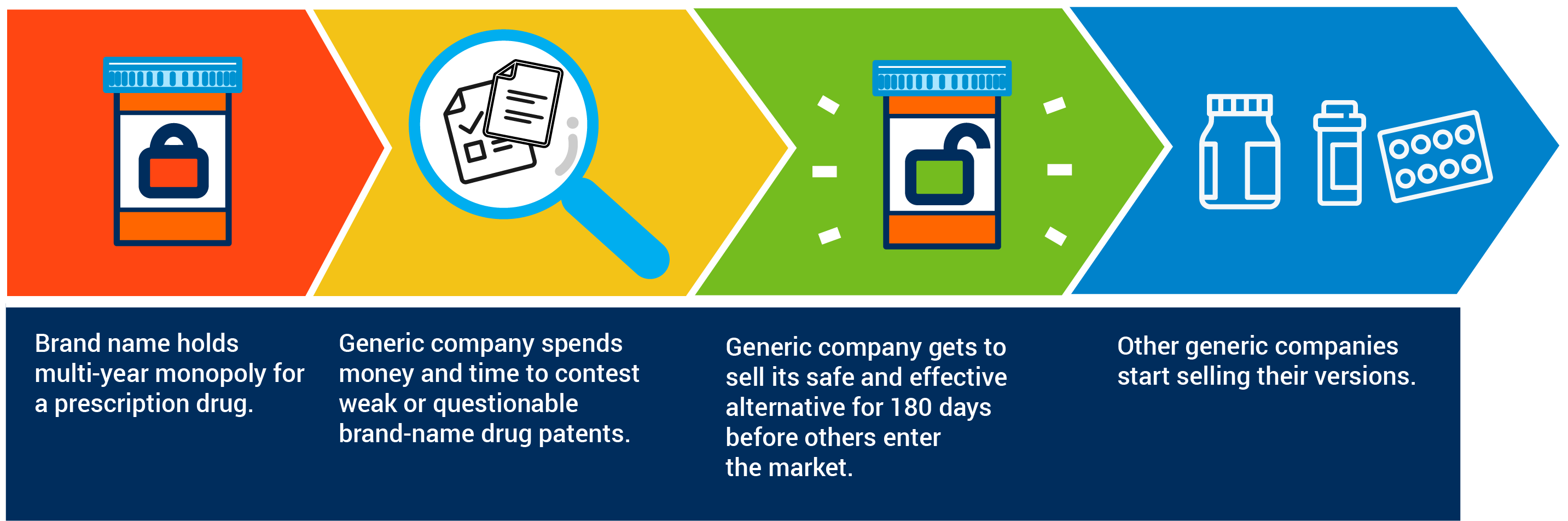
Section 205 threatens to upend this framework by undermining the 180-day exclusivity incentive that drives it. It does this by adding several new conditions that will limit whether generic companies are able to use the 180-day exclusivity to which they are entitled. The new conditions are complex and create significant uncertainty. Moreover, many of them are beyond the control of the generic applicant. The net result is that the “brass ring” of 180-day exclusivity will be tarnished. And this means that fewer generic companies will have a sufficient incentive to take on the risk and expense of challenging brand-name drug companies.
Unfortunately, if enacted, Section 205 will result in higher drug prices for American patients. We should BLOCK Section 205 of the Lower Health Care Costs Act of 2019.
A January 2020 report from Matrix Global Advisors, “The Unintended Economics of the BLOCKING Act,” finds the delay of just one generic medicine will increase prescription drug costs for patients by $1.7 billion.

By Chip Davis, AAM President and CEO
