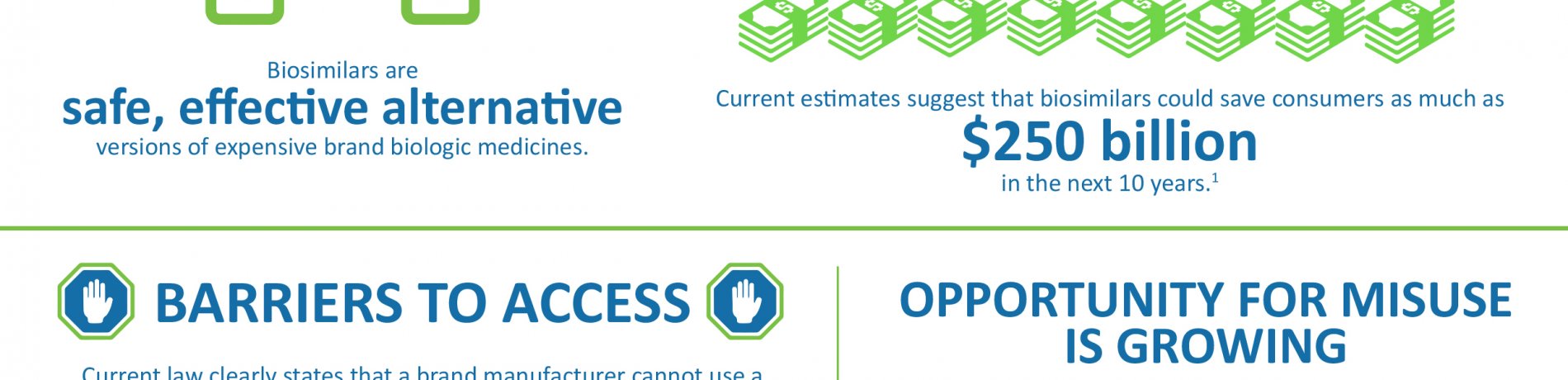
Since it was created in 2007, the Food & Drug Administration’s (FDA’s) Risk Evaluation and Mitigation Strategies (REMS) has been an important tool for patient safety by ensuring that the benefits of a drug or biological product outweigh its safety risks. FDA-mandated REMS serve a clear public health purpose, yet some brand drug companies have been misusing this patient safety program and other restricted access drug programs to extend market monopolies, intentionally limiting patient access to biosimilars. These abuses are growing, resulting in delayed approval of biosimilars, costing patients, the federal government and the health care system billions of dollars.