Congress Must Preserve the 180-Day Incentive for First Generics
In the debate over how to lower the cost of prescription drugs, one thing is certain: Generic competition drives savings for America’s patients and our health care system. The introduction of generic medicines offers patients the same high-quality medicine as the brand-name drug, but at an average price that is 39% lower according to the U.S. Food and Drug Administration (FDA). Prices fall even further as more generics enter the market, and patients benefit when these medicines are readily available.
Notably, the first generic on the market offers the most significant discount off the price of the brand-name drug. A second generic competitor yields an average of 15% in additional savings. With four competitors, the average price falls 19% further. The first generic is thus critical to competition and opens the door for patient savings.
Since 2015, the FDA has approved more than 450 first generics and these medicines comprise about 10% of all generics approved each year. First generics improve patient access to essential treatments and, in recent years, offered more affordable options for patients with multiple sclerosis, asthma, opioid dependence, heart disease, diabetes, cancer, allergies and even the flu. It’s no surprise then that the FDA “considers the approval of the first generic of a brand-name drug to be a public health priority.”
Bringing a first generic to market, however, is not easy or inexpensive. Congress recognized the risks and costs required to develop a first generic by making the first-to-file generic manufacturer eligible for a 180-day exclusivity period. This 180-day incentive is critical in the decision-making process of generic manufacturers when determining which medicines to bring to market and without it patient access to first generics would be delayed for years.
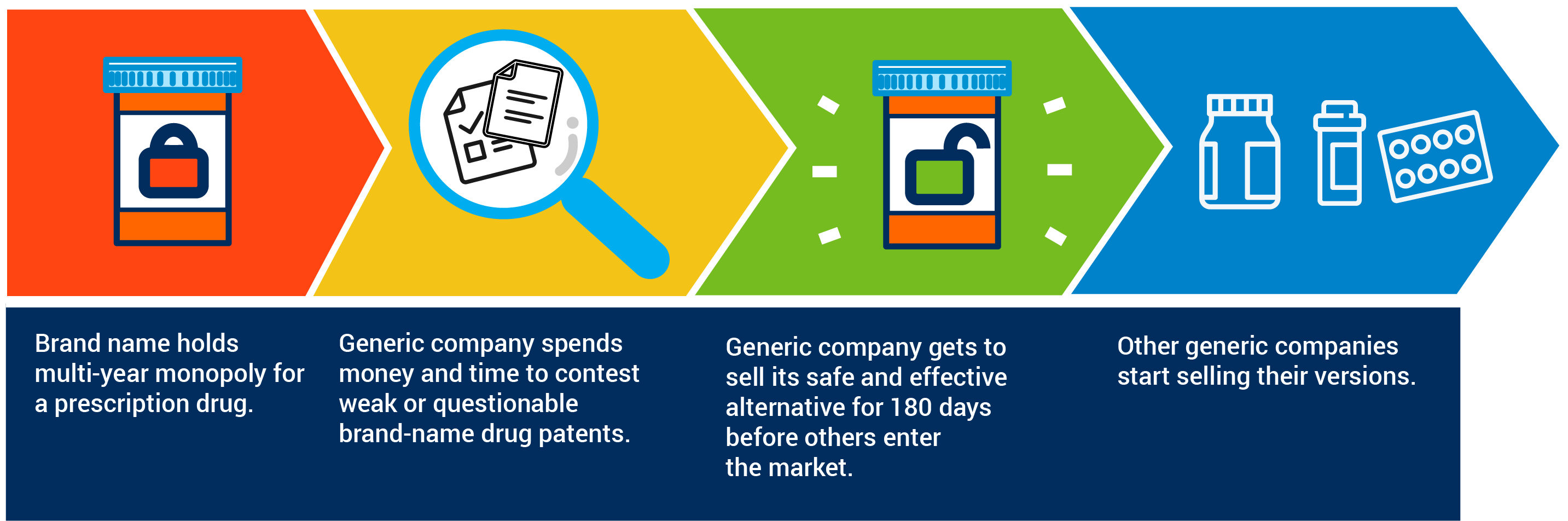
The 180-Day Exclusivity Rule Supports Generic Competition. Here’s How.
Unfortunately, Congress is considering changes to the 180-day incentive for first generics in the form of The BLOCKING Act (H.R. 938/S. 1895). A recent study found The BLOCKING Act would result in increased spending on brand-name drugs by an average of $1.7 billion for each generic delayed. At a time of record FDA approvals of generic drugs, The BLOCKING Act would undo the progress made in recent years to increase patient access to first generics. This simply doesn’t add up.
Americans are struggling with the high cost of prescription drugs and more should be done to lower the cost of medicines at the pharmacy counter. Increasing patient access to first generics is a proven, market-based solution that relies on competitive forces to lower drug prices. Congress should preserve the 180-day incentive and its 35-year track record of success and not weaken it as The BLOCKING Act proposes to do.

By Erik Komendant, AAM Vice President, Federal Government Affairs

