In 2021, employers experienced a 6.3% jump in health benefit costs.1 Findings such as these, paired with high brand-name drug costs, are why commercial and employer-sponsored health plans have long looked forward to savings from biosimilar competition. Unfortunately, employers soon will be facing higher costs, if provisions in the Build Back Better Act (BBB) that discourage biosimilar development are adopted by Congress.
Biosimilar medicines are lower-cost versions of the high-priced specialty medicines that account for 53% of all pharmaceutical spending.2 Biosimilars, on average, are priced at less than half that of their brand competitor at the time of launch. Moreover, the competition they bring forces brand drugs to reduce their prices by, on average, 28%. The competition biosimilars bring to market generates savings for the US healthcare system that are projected to exceed $130 billion by 2025.3 These savings are critically important to bending the drug cost curve downward for the 152 million patients covered by commercial plans in the United States. For instance, biosimilar competition in oncology helped reduce spending growth by nearly half in one year alone.4
And the biosimilar pipeline continues to grow, with more than 95 products currently under development, a 50% increase in just two years.5
But the Build Back Better proposal directing the federal government to dictate prices for 100 drugs or more by 2031 undermines this progress and the promise of future savings, particularly for employers and patients in commercial health insurance markets.
In fact, if the proposal is enacted, it is likely that many of the biosimilars in development will no longer be commercially viable as a result of the significant uncertainty created by the BBB. This in turn would not only result in less savings to Medicare but would deprive patients and payers in the commercial market of significant savings and leave them dependent on high-cost brands.
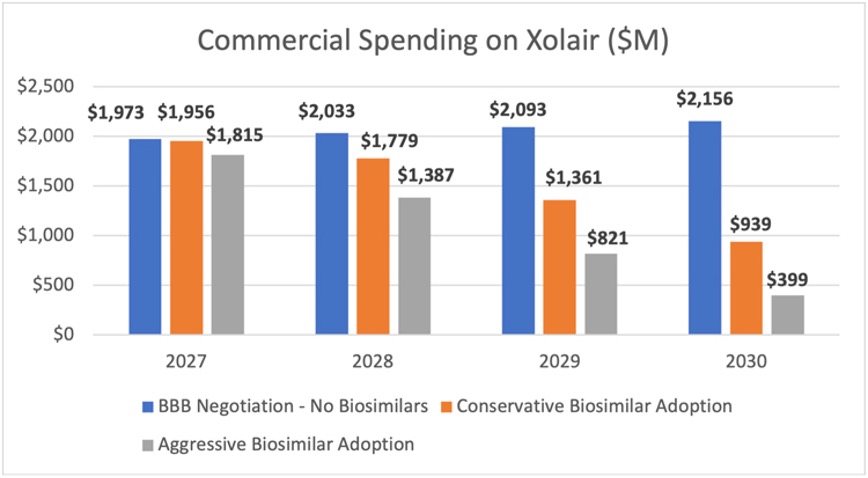
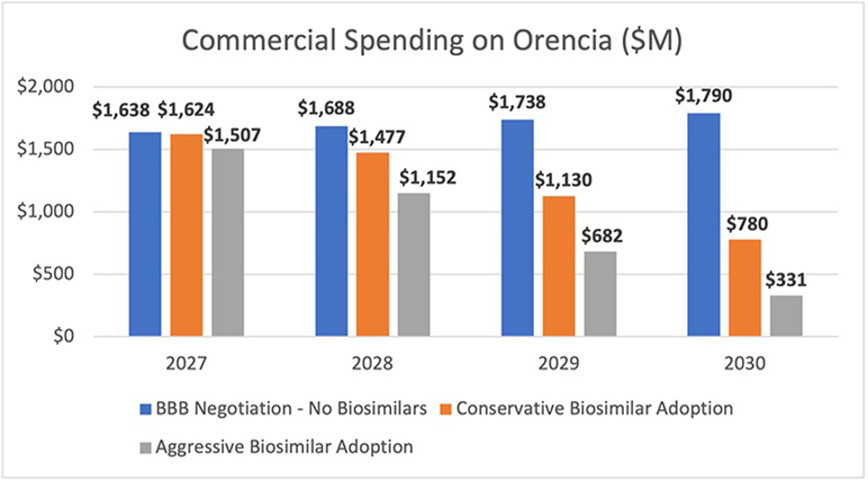
AAM’s Biosimilars Council assessed the potential lost savings to the commercial market using Xolair (omalizumab) and Orencia (abatacept) as examples. Used to treat asthma and rheumatoid arthritis, respectively, as well as other conditions, each of these products has total spending of around $2 billion.6
Analyzing a nearly identical proposal in an earlier version of the Build Back Better Act that subjected these products to negotiation beginning in 2027 (now moved to 2028), the analysis found Medicare would save 60% - $309.8 million and $750.4 million each year - through lower spending on Xolair and Orencia, respectively. But the market uncertainty created by the legislation could undermine the willingness of biosimilar developers to invest the $100-$250 million and 8-12 years necessary to bring a lower-cost option to market.
The Biosimilars Council estimated the impact of the lack of biosimilar competition for these two products across a range of scenarios that included the average biosimilar market share for current biosimilar products and a more aggressive scenario that anticipates biosimilar adoption will continue to progress this decade. We found that the legislation would cause patients and employers in the commercial insurance market to spend between $1.8 and $3.2 billion more on Orencia between 2027 and 2030 in the absence of biosimilar competition. For Xolair, the commercial market would spend between $2.2 and $3.8 billion more than it would if there were biosimilar competition.
Altogether, this would mean lost savings of between $4 billion and $7 billion between 2027-2030 for just two products. These lost savings will mean higher patient out of pocket costs and higher insurance premiums for employers and employees.
The BBB represents a misguided approach to reducing drug spending and would harm long-term opportunities for greater savings through the market-based competition that biosimilars and generics offer. Patients deserve access to more high-quality products, rather than fewer, and payers will be able to save money in an environment with more competition rather than less. The BBB scheme would leave many patients and the health care system overall worse off with fewer options for care and higher costs. Congress should instead focus on removing anticompetitive patent and market barriers to competition in order to bring lower-priced biosimilar and generic competition to market as early as possible. This approach would improve competition, save payer’s money, and improve access to life-saving therapies for American patients.
Share Your Voice
Tell Congress to protect access to your safe, affordable generic and biosimilar medicines.
By Craig Burton, Senior Vice President, Policy and Strategic Alliances
Executive Director, Biosimilars Council
Published on July 20, 2022
References
- Mercer. (December 2021). National Survey of Employer-Sponsored Health Plans. (Link)
- IQVIA. (May 2021). The Use of Medicines in the U.S.: Spending and Usage Trends and Outlook to 2025. (Link).
- Ibid.
- FDA-TRACK: Center for Drug Evaluation & Research - Pre-Approval Safety Review - Biosimilars Dashboard (Link).
- IQVIA. (May 2021). The Use of Medicines in the U.S.: Spending and Usage Trends and Outlook to 2025 (Link).
- IQVIA. (October 2020). Biosimilars in the United States 2020–2024 Competition, Savings, and Sustainability. (Link).