Just as FDA plays a vital role through the review and approval of more affordable generic and biosimilar drugs, the Centers for Medicare & Medicaid Services (CMS) affects patient access to these lower priced medicines through its ability to influence drug formulary design in Medicare and Medicaid.
In order to meet its goal of lowering patient out-of-pocket costs, it is essential that the Administration work to ensure federal programs encourage the use of lower-cost therapeutically equivalent generics and biosimilars. Accordingly, we asked CMS to include the following common sense policies as part of its “Call Letter” for CY2020 (CMS-4180-P):
- require plans to automatically include generic and biosimilar medicines on generic formulary tiers immediately after launch;
- ensure plans place generics on generic tiers and brands on brand formulary tiers to reverse the practice of mixing generics on brand tiers; and
- create a dedicated, more favorable specialty tier with lower beneficiary cost-sharing for generics and biosimilars.
Bipartisan Congressional Letter to HHS and CMS
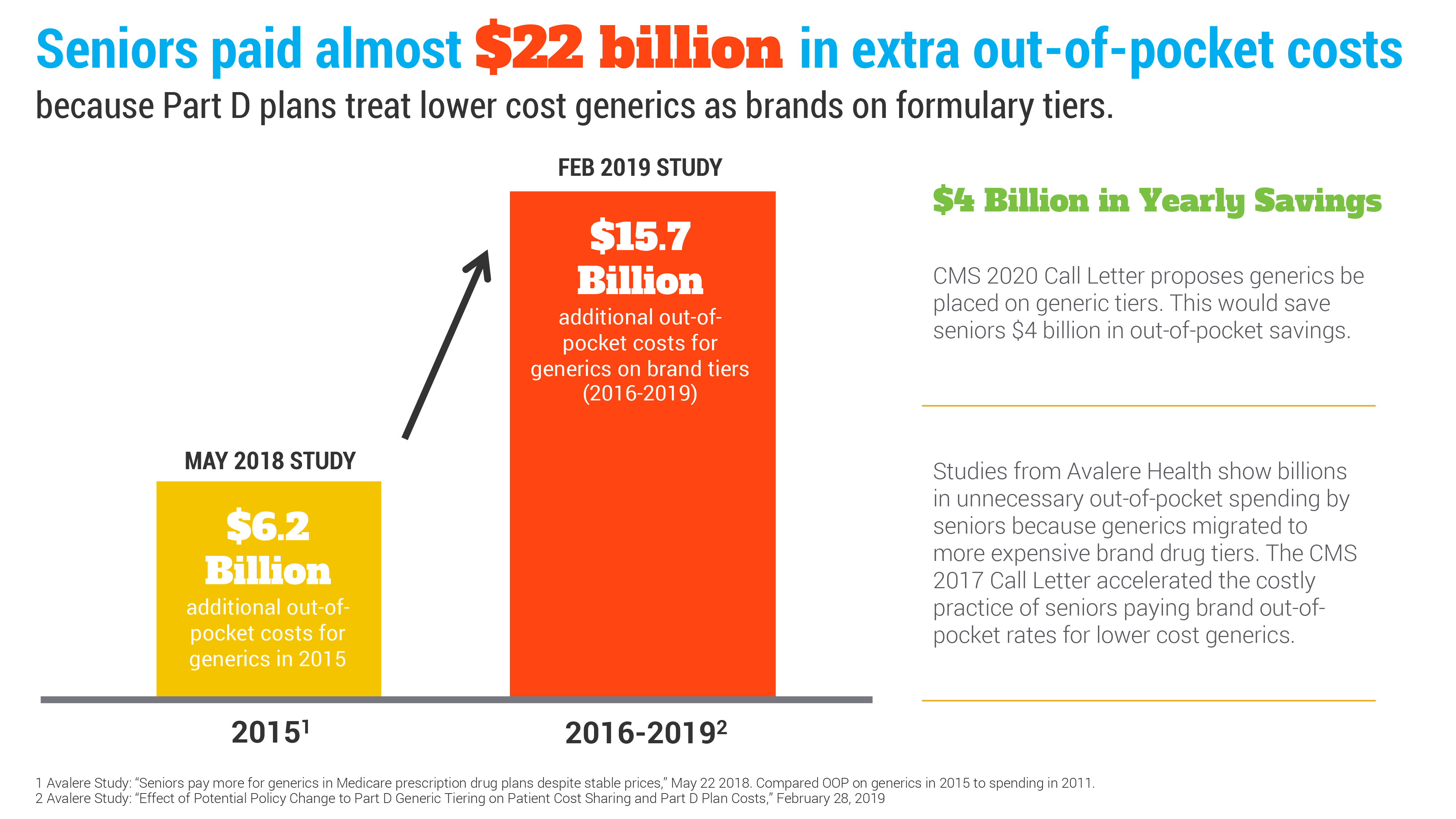
A new report from Avalere Health, provides further evidence that America’s patients are needlessly spending too much out-of-pocket for affordable generics. Since 2015, seniors have paid nearly $22 billion in additional out-of-pocket costs for their prescription drugs in Medicare. Enacting AAM’s reforms can place $4 billion back in seniors' pockets each year.
Learn how your generic and biosimilar industry is committed to your health.