AAM’s annual savings report, in partnership with the IQVIA Institute, reveals that Food & Drug Administration (FDA) approved generic and biosimilar medicines created $467 billion in savings in 2024 for patients and the U.S. healthcare system, and $3.4 trillion in savings over the last ten years. Savings from biosimilar medicines alone increased to $20.2 billion in 2024 and $56.2 billion since the first biosimilar entry in 2015.
However, the long-term outlook for America’s patients hinges on addressing barriers to the development and adoption of lower-cost medicines. Without action to strengthen generic and biosimilar markets, many lower-cost medicines may fall into shortage or stop being marketed in the U.S. altogether.
“Generic medicines save money,” said John Murphy III, President and CEO of AAM. “Our industry is the only sector that consistently reduces spending across the U.S. healthcare ecosystem. Yet little is being done to support sustainability in the generic and biosimilar marketplace. Decades of price deflation can create unsustainable market conditions, threatening patient care and increasing the risk of shortages or market exits. Policymakers must streamline FDA processes, curb patent abuse, stop PBMs and Medicare policies from denying patient access, and roll back harmful federal policies, including IRA price controls.”
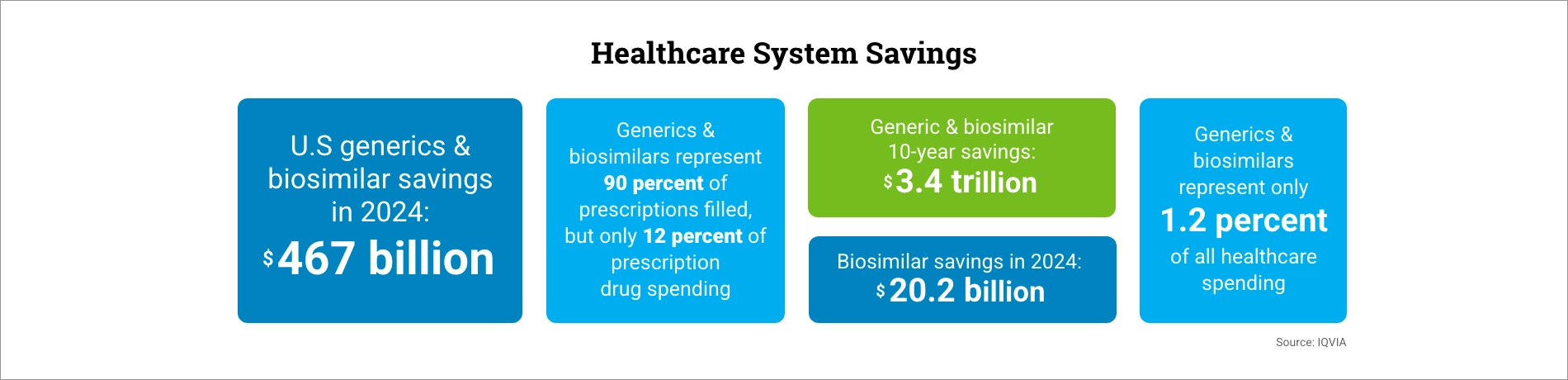
This represents an increase from 2023, which found $445 billion in generic and biosimilar savings, underscoring continued cost relief for patients, employers, and taxpayers.
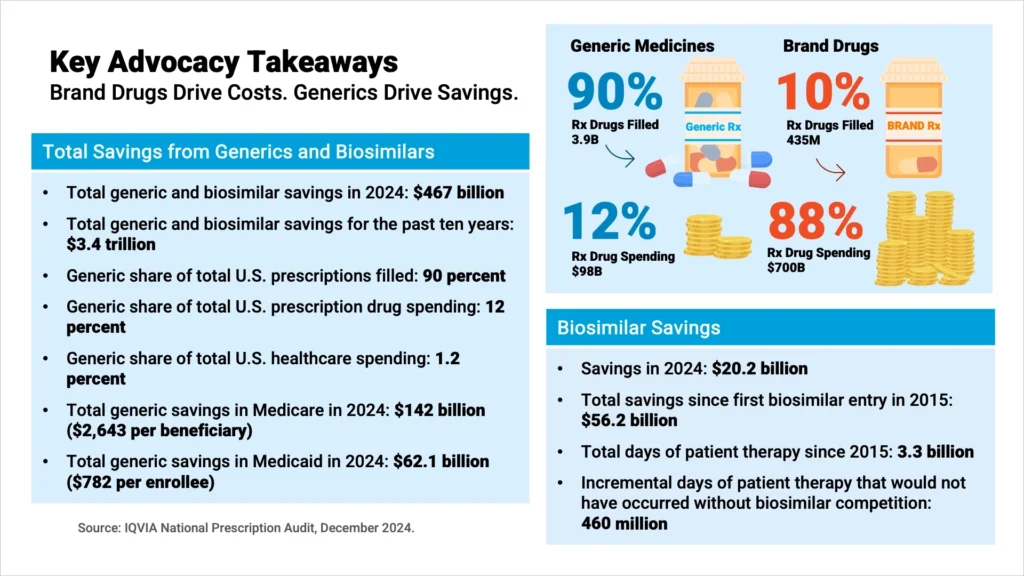
In 2024, generics accounted for 90% of all prescriptions filled in the U.S., but only 12% of total prescription drug spending. Brand drugs represented just 10% of prescriptions while accounting for 88% of spending. That year, Americans filled 3.9 billion generic prescriptions compared to 435 million brand prescriptions, spending $98 billion on generics versus $700 billion on brand drugs.
Generics and biosimilars continue to deliver critical savings across the healthcare system, particularly for Medicare and employer-sponsored insurance. Despite these savings, many patients still pay too much for generic prescriptions.
Since 2015, biosimilars have been used in approximately 3.3 billion days of patient therapy with no unique clinical challenges. Competition has supported more than 460 million incremental days of therapy that patients otherwise would not have received. However, with 90% of brand-name biologics losing patent protection in the next decade and no biosimilar competition currently in development, a projected $234 billion in potential savings is at risk. Policymakers must act now to eliminate unnecessary regulatory barriers, end patent thickets, ensure patient access, and reverse the damage caused by IRA price controls.
Learn More on the Biosimilars Council Website