Last week, AAM launched a new event—and a new tradition. GRx+Biosims welcomed nearly 550 participants from government, industry and related sectors to Baltimore, Maryland for presentations, workshops and high-level dialogues about the dynamic landscape for generic and biosimilar drugs.
Hosted at the Baltimore Hilton September 5-7, the event encompassed the full range of AAM’s mission of improving access to safe, quality and effective medicine—focusing specifically on better understanding how science, business and policy intersect in the approval of small-molecule generics, complex generics and biosimilars. As AAM President and CEO Chip Davis put it in his remarks, “GRx+Biosims, that is who we are. That is why we are all here. If it can be substituted and save consumers, patients and payers money in the process… that’s us.”
After Maryland Governor Larry Hogan delivered a welcome address by video, hailing the role that generics and biosimilars play in the U.S. health care system, Centers for Medicare and Medicaid Services Administrator Seema Verma addressed the gathering. She pointed to a recent study finding that Medicare Part D plans spent almost $9 billion on brand-name drugs when therapeutically equivalent generics were available. “Lowering the cost of prescription drugs isn’t just something we’d like to do,” she stated. “It’s something we must do.” She gave an overview of efforts to encourage uptake of generic drugs and to promote biosimilars and highlighted aspects of the Trump Administration’s drug pricing blueprint.
FDA Deputy Commissioner for Policy, Planning, Legislation and Analysis Anna Abram underscored the need to balance innovation and competition in the pharmaceutical industry. The FDA’s Drug Competition Action Plan, she said, was introduced to “further encourage competition and help bring greater efficiency and transparency to the generic drug approval process.”
“It was important that we not sacrifice the scientific rigor underlying our generic drug program in the process,” she added. “After all, achieving 90 percent of prescription drugs dispensed represents years of hard work building confidence in generic drugs. Maintaining that confidence must remain paramount to the FDA, as it must to all of you.” Ms. Abram also highlighted joint efforts of the FDA and FTC to combat tactics that brand-name companies deploy to prevent generic developers from obtaining the samples of brand drugs.
Attendees from member companies and beyond responded positively to AAM’s creation of GRx+Biosims, an event combining three existing meetings—Leading on Biosimilars, Fall Tech and the AAM CMC Workshop. Networking and socializing continued alongside workshops and breakout sessions on a range of issues.
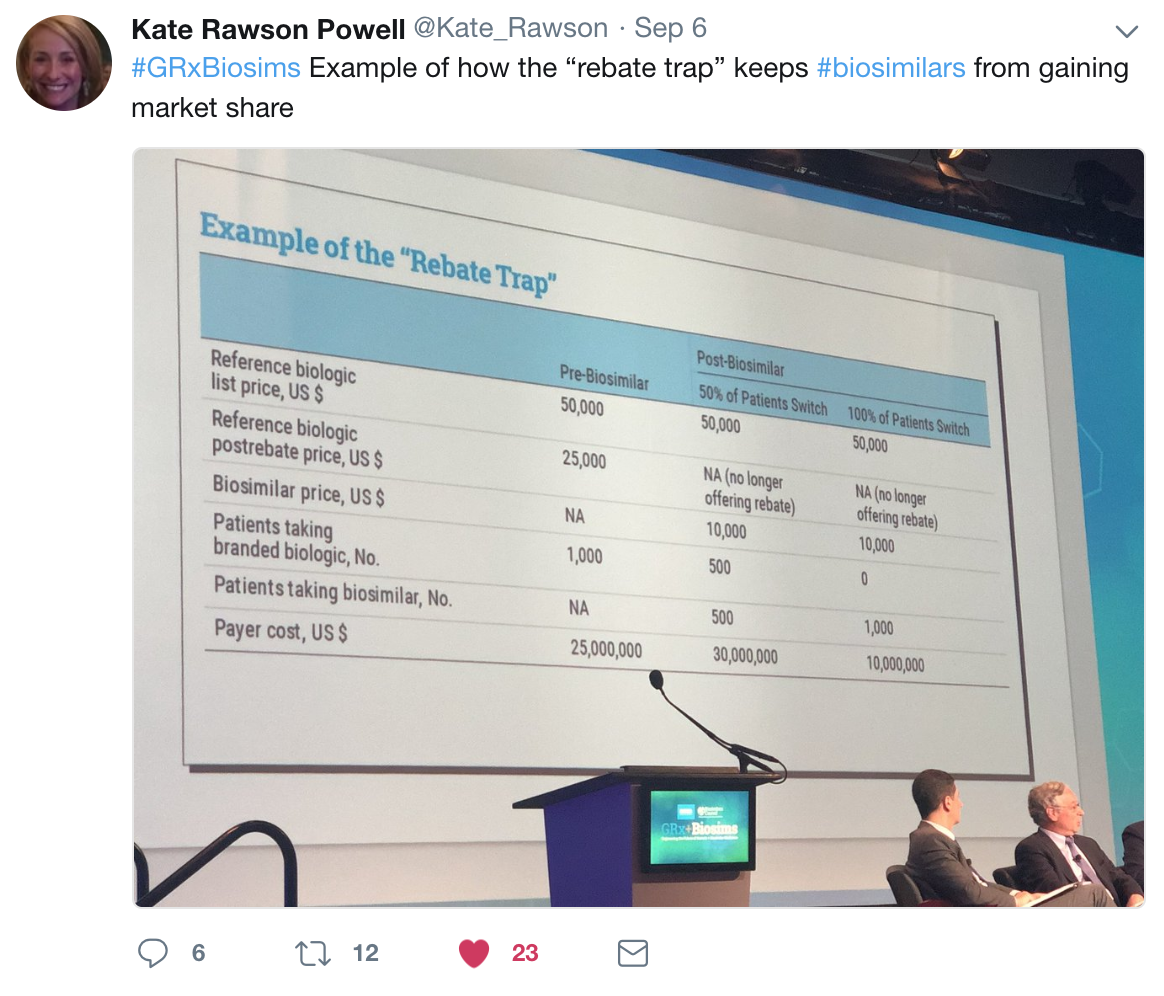 |
 |
 |
 |
Next month, AAM offers another opportunity to learn and network with Next Steps: Manufacturing and Quality Workshop, an event dedicated to the science, manufacturing and quality components of generics and biosimilars. Experts from USP, FDA, industry and academia will be on hand in North Bethesda, Maryland October 22-23.
Access! 2019, AAM’s annual meeting, comes to New Orleans February 4-6.

By Rachel Schwartz, AAM Communications Director
