Next week, join us on November 4-6, 2019 in North Bethesda, Maryland for the premier scientific and regulatory event for the U.S. generics and biosimilars industries. AAM and the Biosimilars Council have planned timely programming relevant to technical, regulatory, policy and legal professionals like you.
There’s still time to register and hear from top-in-their-field officials and subject matter experts; share knowledge and best practices; and gain insights on navigating the regulatory process approvals and the evolving policy landscape.
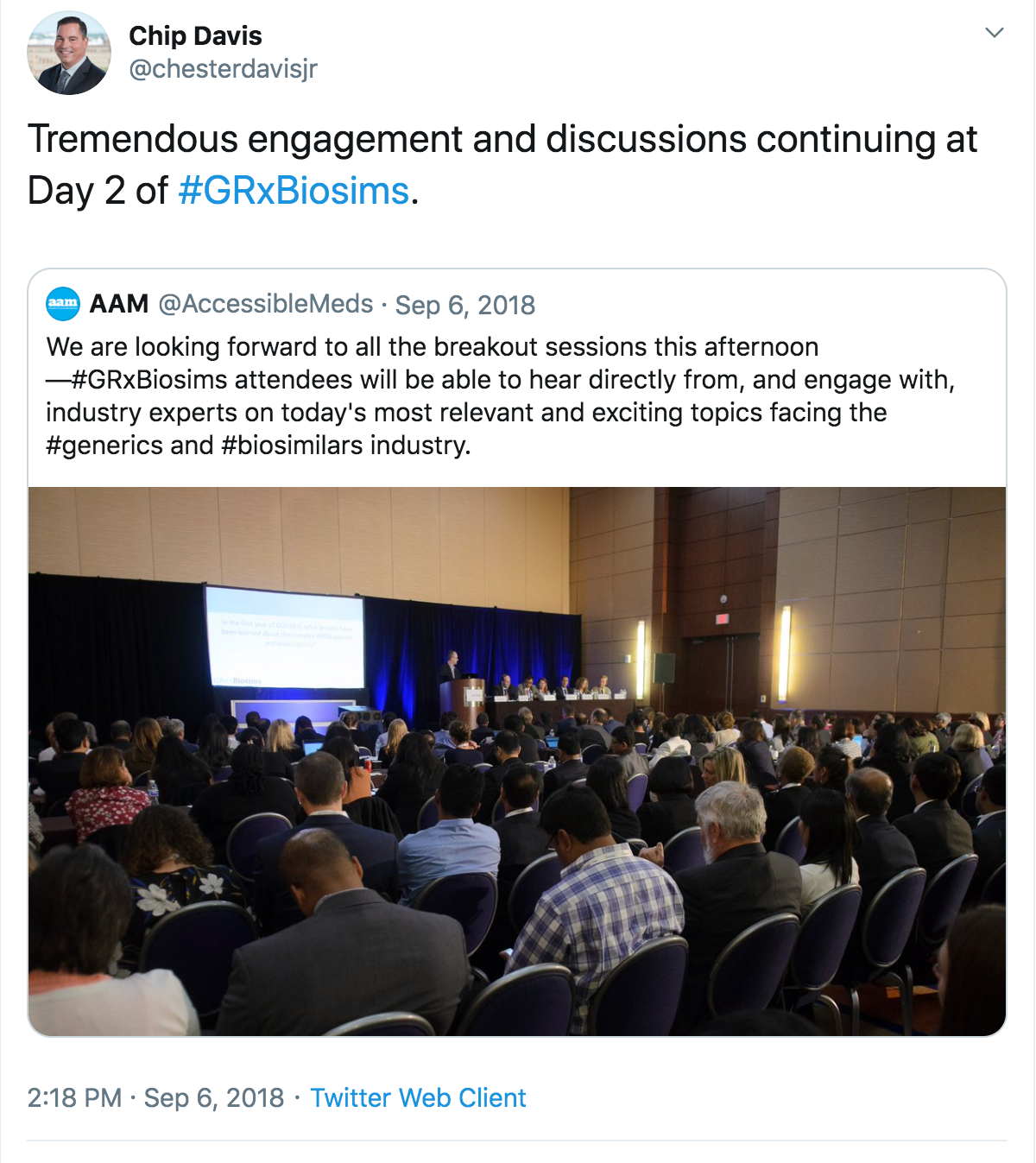
The packed agenda includes more than 165 speakers from industry, academia and FDA, along with plenty of opportunities for networking.
- November 4 highlights include remarks by Norman (Ned) Sharpless, M.D. (Acting Commissioner, FDA), Stacy Cline Amin, JD (Chief Counsel, Office of the Chief Counsel, FDA) and Sarah Yim, M.D. (Acting Director for Therapeutic Biologics, Office of New Drugs, Therapeutic Biologics and Biosimilars Team, CDER, FDA). View Day 1 agenda here.
- November 5 highlights include remarks by Alonza Cruse (Director, Office of Pharmaceutical Quality Operations, Office of Regulatory Affairs, CDER, FDA) and Patrizia Cavazzoni, MD (Deputy Director for Operations, CDER, FDA) and the Exposition. View Day 2 agenda here.
- November 6 highlights include remarks by Scott Gottlieb, M.D. (Former Commissioner, FDA) and the Complex Product Workshop, which is available only to registered GRx+Biosims attendees; separate registration is required at no additional fee. View Day 3 agenda here.
Use #GRxBiosims on social media and tell your colleagues why you’re attending this year.
 |
 |
 |
 |
 |
 |

By Jennifer Soup, AAM Director of Meetings & Marketing

