Second in a series, view first.
We are all experiencing the impact of the novel coronavirus (COVID-19) pandemic. From the disruption of our normal routines to the stresses imposed on our health care system, we are learning to adjust, prepare our families as best we can and help those in need.
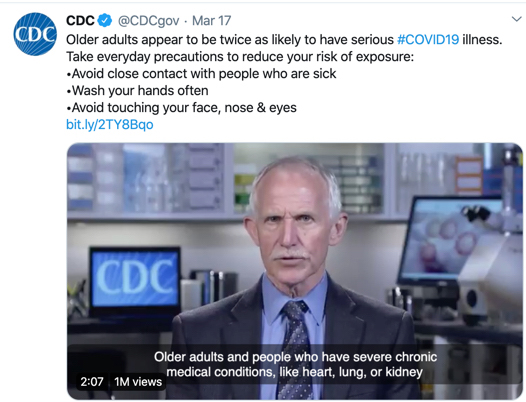
One of the preparedness recommendations from the Centers for Disease Control and independent sources such as Consumer Reports is to stock up on needed prescriptions and over-the-counter medications. Patients with chronic conditions and older Americans should especially take heed of this recommendation. The amount of supply will vary by the type of prescription, so it’s best to consult with your physician and pharmacist on the appropriate quantity.
Because generic medicines account for 90% of all prescriptions filled, AAM’s member companies understand the vital role our industry plays in making sure patients can continue to access essential medicines. Our members are working around the clock to ensure health care workers on the front lines and pharmacies across the country are able to provide the medicines patients need for their health. While press reports indicate the wait at the pharmacy may be longer and demand for mail order prescriptions has increased, patients are able to get the generic medicines they need.

|
|
 |
 |
While the National Institutes of Health and others are working to develop innovative vaccines and treatments for COVID-19, our members are partnering with health organizations and governments around the world to evaluate the use of currently available generic medicines for the treatment of patients with COVID-19. As more research is done, the CDC will continue to be an excellent source for the latest guidelines on the clinical treatment and care for COVID-19. Some of our members also manufacture over-the-counter medicines used to help treat the symptoms of the illness. For example, generic acetaminophen can help reduce a patient’s fever.
COVID-19 is presenting the global health community with new challenges. While the response evolves daily, AAM and its members are committed to meeting the health care needs of America’s patients. AAM will continue to work with our companies, government regulators and other stakeholders to help ensure a steady supply of generic and biosimilar medicines during this public health emergency.
View next in the series: COVID-19: This Week’s Initiatives From the Generic and Biosimilars Industry

Jeff Francer, AAM Interim CEO and General Counsel
Published on March 19, 2020

