On Wednesday, David Gaugh, R.Ph., AAM’s Senior Vice President, Sciences & Regulatory Affairs, provided testimony to the House Energy and Commerce Health Subcommittee hearing, “Safeguarding Pharmaceutical Supply Chains in a Global Economy.” Here are five important takeaways from his remarks.
- Patient safety is the number one priority for AAM and its member companies. Generics and biosimilars are just as safe and effective as their brand-name drug counterparts. And the FDA would not approve generics if they were not safe and effective treatments.
- Generics and biosimilars are integral to patient health. Generic medicines play an integral role in health care and enhance patient access to life-saving treatments. Over the last 10 years, generic manufacturers have delivered savings of nearly $2 trillion – including $293 billion in 2018 – to patients and the health care system. Experts estimate that FDA-approved biosimilars could save more than $54 billion over the next 10 years. (See RAND, “Biosimilars Cost Savings in the United States,” October 2017.)
Download 2019 Access & Savings Report - Generics and biosimilars are part of the same global pharmaceutical supply chain as the one for brand-name drugs and biologics. The FDA ensures all pharmaceuticals meet the same high-quality standards regardless of where brand-name drugs, biologics, generics and biosimilars are manufactured. All pharmaceuticals, whether generic or brand, must be manufactured in accordance with rigorous regulatory standards that require high levels of diligence and accompanying documentation.
- We remain committed to ensuring the FDA continues to have the resources to perform thorough inspections of facilities. Generic Drug User Fee Amendments (GDUFA) and its reauthorization in 2017 included a $4 billion commitment from the generic drug industry. We are pleased that the number of FDA’s foreign inspections continues to rise, in no small part based on funding provided by AAM’s member companies through GDUFA and the Biosimilars User Fee Act (BsUFA).
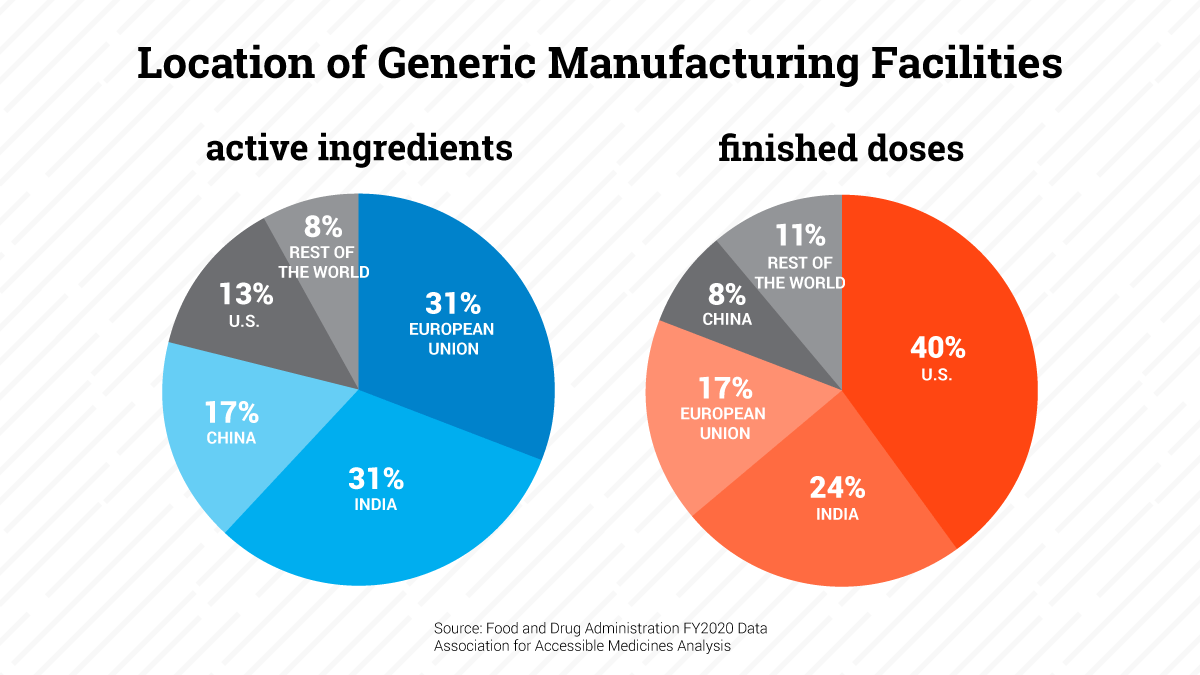
- On the global supply chain, facts matter. We understand why there would be concerns about recently reported data that paints a distorted picture of a global supply chain that is heavily reliant on China and other countries for API. We testified to help set the record straight and provide more accurate analysis of where generic active pharmaceutical ingredients (API) and finished dosage form (FDF) facilities are located.

By Rachel Schwartz, AAM Communications Director

