When a brand-name prescription drug’s market exclusivity (its monopoly) and patents expire, generic and biosimilar medicines become available, the savings begin and patients win. That’s how the system is supposed to work. However, brand-name pharmaceutical companies have increasingly abused FDA’s safety programs (Risk Evaluation and Mitigation Strategies, or REMS) and other manufacturer-imposed restricted distribution of samples to delay competition and impede patient access to more affordable prescription drugs.
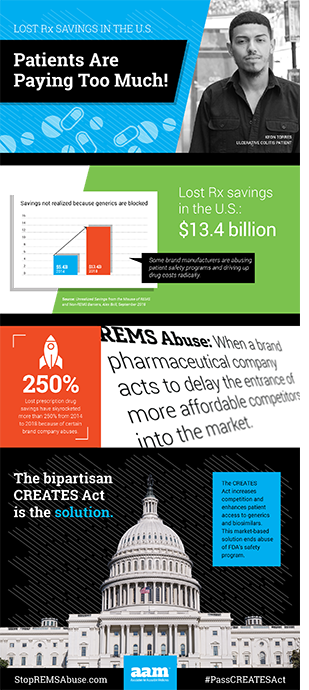
The result, according to a recent study by Alex Brill of Matrix Global Advisors, is $13.4 billion a year in unnecessary drug spending. We all pay more, as patients and taxpayers, and the cost impact is growing.
Before a deep dive into the games played, it’s important to remember a key aspect of how more-affordable generics reach the pharmacy. Generic manufacturers are required to test their generic formulation against the original (or reference) brand drug, which requires access to a sample of the brand product. For more than 30 years since the enactment of the 1984 Hatch-Waxman Act, selling samples to generic manufacturers was standard practice.
Over the years, competition from more affordable, FDA-approved generics made possible by Hatch-Waxman saved patients trillions of dollars. In 2017, savings amounted to $265 billion.
So how are brand-name pharmaceutical companies gaming the rules and preventing patients from saving more?
In 2007, the FDA required new drug safety programs, known as REMS, for certain pharmaceuticals. These measures were intended to ensure that risks are appropriately communicated to patients, health care providers and pharmacists. They were not intended – and Congress passed language stating as much – to interfere with the ability of generic manufacturers to purchase brand drug samples. Unfortunately, some brand-name pharmaceutical companies abused these new FDA rules to block the sale of samples as an anti-competitive tactic.
As early as 2013, the Federal Trade Commission (FTC) took note of this practice, stating, “If brand firms are able to block generic competition by denying access to the product samples needed to obtain FDA approval, this conduct may prevent the Hatch-Waxman framework from functioning as Congress intended.”
FDA has issued similar objections and taken action to limit abuse of its safety programs. Last year, FDA Commissioner Scott Gottlieb, MD, said, “Branded companies’ use of REMS – which FDA adopts as a way to ensure the safe use of certain drugs – is also sometimes being used as a way to frustrate the ability of generic firms to purchase the doses of a branded drug that they need to run their studies. This needs to stop.”
Despite FDA’s actions and warnings, however, abuse of the agency’s safety programs and restricted distribution schemes continue. It has become clear the agency needs new authorities to fully stop these abusive practices.
Over the last four years, the cost to patients and taxpayers in unrealized savings from REMS and restricted access abuse spiked by 250 percent. Brill notes, “Government, consumers and private payors are missing out on sizeable health care savings from the misuse of REMS programs.” Moreover, he adds, the promise of biosimilar drugs is being stifled because biologics are “ripe for the type of restricted access misuse.”
These brand “shenanigans” are costing patients and taxpayers more than ever. Fortunately, there is a solution to this $13.4 billion annual problem – passage of the Creating and Restoring Equal Access to Equivalent Samples (CREATES) Act.
The CREATES Act has bipartisan support on Capitol Hill and across the country. Close to 90 organizations have endorsed the CREATES Act, and the Senate Judiciary Committee advanced this solution by a vote of 15-6 in June.
In the words of FDA Commissioner Gottlieb, it’s time to end the shenanigans. Join the effort to increase competition and pass the CREATES Act. Patients benefit from lower prescription drug costs, and we all win when our nation is healthier.