At a time when public opinion is often divided, Americans are remarkably aligned on two things: prescription drug prices as a whole are too high, and generic medicines bring lower prices.
This is backed up by the facts. The use of generic and biosimilar medicines has generated more than $3.1 trillion in savings to U.S. patients and the healthcare system over the past ten years – more than $445 billion in 2023 alone, including more than $137 billion in savings for the Medicare program.
Why then does the Medicare program consistently see slower adoption of new lower cost generic and biosimilar medicines when these would result in lower patient out of pocket costs and reduced taxpayer spending?
As AAM has previously described, the adoption of new generic medicines – once the backbone of savings for patients and taxpayers – is slowing. And Medicare plans have been slow to cover biosimilars that are 95 percent lower priced than their reference products; instead, the plans have shifted more patients to new brand drugs with higher prices. This is the result of Medicare incentives rewarding middlemen like pharmacy benefit managers and health plans for use of high-priced brand drugs with high rebates – even though this causes patients to pay more at the pharmacy counter.
Beginning this year, the Medicare drug program underwent significant changes that were arguably intended to change those PBM and plan incentives and encourage greater use of lower cost generics and biosimilars.
Unfortunately, the Medicare drug “redesign” left in place many of the same perverse incentives, including PBM reliance on rebates and fees on high-priced brands. And new data reveals that Medicare plans continue to block or delay coverage of new generics, even under the redesigned benefit.
On behalf of AAM, health consulting firm Avalere examined Part D payer coverage rates for new generics launched between 2016 and 2025. Historical models show Part D plan sponsors coverage rates for first generics lag well behind commercial plan coverage of the same drug products. For example, it appears to take roughly three years before new generics are covered by more than half of all Medicare drugs plans. On the other hand, on average, at least 50 percent of non-Medicare commercial plans typically cover first generic drugs within the year after launch.
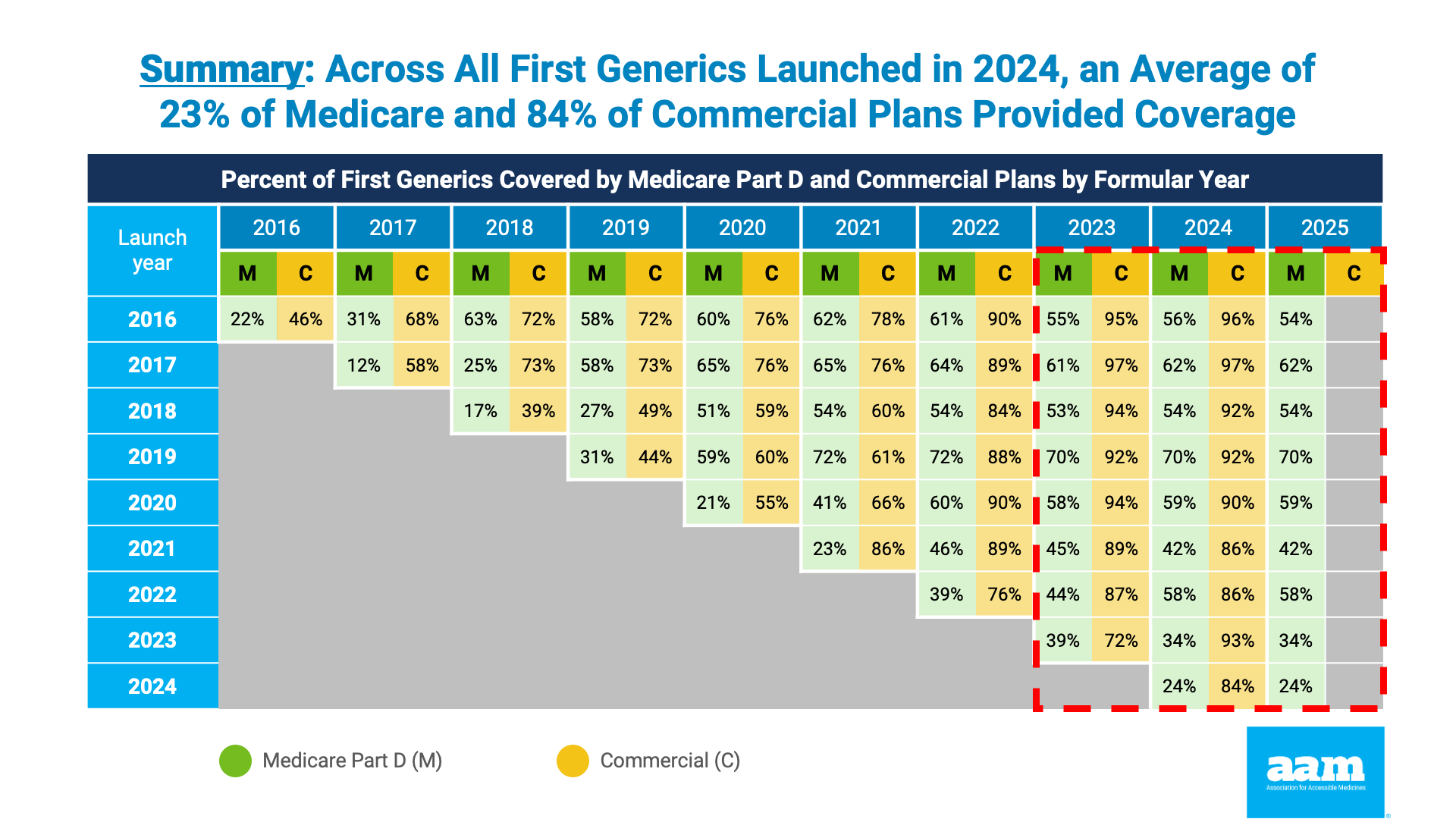
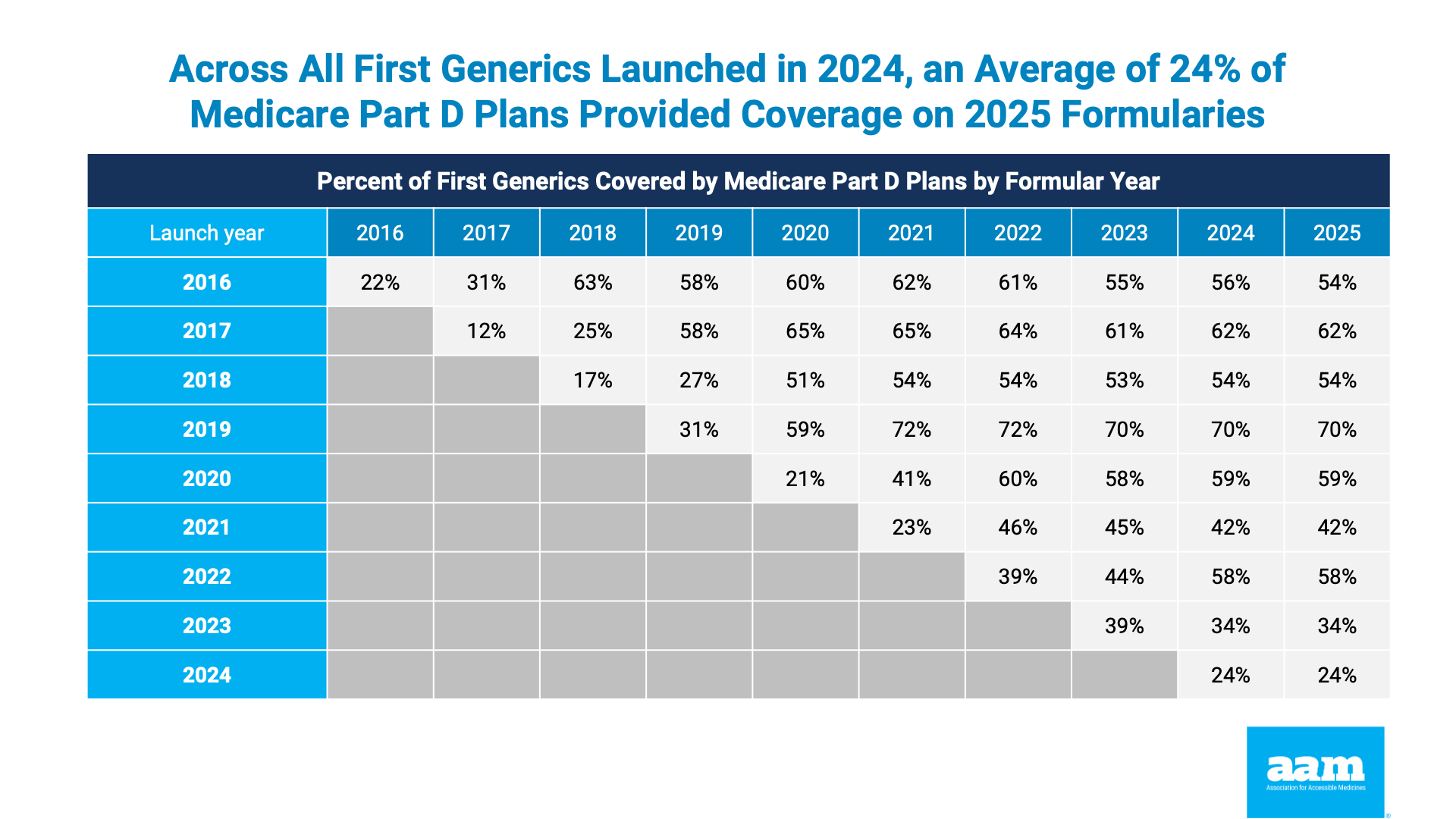
Although the Part D redesign was framed as seeking to encourage faster Medicare adoption of new generics, the updated analysis released today shows no improvement. In fact, the rate of new generic drug coverage actually declined in some cases. Across all first generics launched in 2024, an average of 24 percent of Medicare plans provided coverage in 2025. Even for first generic drugs launched in 2021, the coverage rate among Part D plans was limited to 42 percent. The data are clear: Part D enrollees are being denied access to new lower price generic medicines.
This is the result of a Medicare drug program that continues to permit and reward PBM and plan formulary practices that favor use of higher cost medicines, including through rebates and fees.
Today, AAM submitted comments to CMS, the agency charged with administering the Medicare drug benefit, highlighting the extent and ways in which “Part D sponsors and their PBMs engage in practices that favor, intentionally or unintentionally, more expensive reference drugs and biological products over generic drugs, biosimilar products, and other lower cost Part D drugs in terms of formulary placement or non-placement.”
AAM notes that these PBM formulary and utilization management decisions not only delay coverage of new generic and biosimilar medicines, but also perversely result in unnecessary increases in patient out-of-pocket costs.
AAM encourages CMS to implement policies that will result in lower taxpayer spending and reduced patient costs through the use of lower-cost generics and biosimilars first. As part of this, any Medicare drug plan covering a brand drug instead of a generic or biosimilar should be required to prove to CMS that the net cost at the unit level of the brand is less than that of the generic or biosimilar.
The perverse incentives and distortionary effects of these plan and PBM coverage decisions are well-established. Rather than continuing to permit the use of high-cost brand products over new generics or biosimilars, CMS should enact common sense policies encouraging the use of lower cost medicines. This aligns with the goal of improving access to covered, lower-cost medicines and, by extension, encourages beneficiary enrollment in the Part D program.
Generic and biosimilar medicines are the backbone of accessible and efficient health care for America’s patients and taxpayers. As the Trump Administration moves forward, it has a critical opportunity to improve the efficiency and fiscal sustainability of the Medicare program, reduce taxpayer spending, and reduce out-of-pocket costs for millions of Medicare enrollees by ensuring that America’s patients have access to generics and biosimilars first.

