The Facts About the Generic Supply and Production of Antibiotics Used to Treat Flu, RSV and COVID
Families and physicians in certain parts of the country are finding it difficult to access commonly prescribed medicines, such as amoxicillin, this winter. The combination of widespread influenza, respiratory syncytial virus (RSV) and COVID infections – in what public health experts are calling a tripledemic
– has led to unforeseen demand for these low-cost generic treatments. In response, generic manufacturers are rapidly increasing production and doing their part to help ensure patients can fill their prescriptions.
Demand went up this year, they [drug manufacturers] anticipated some increase in demand, but not as much as we’re seeing and not this early in the season. So, it’s not any kind of disruption in supply.
—Scott Gottlieb, Former Commissioner of the U.S. Food and Drug Administration
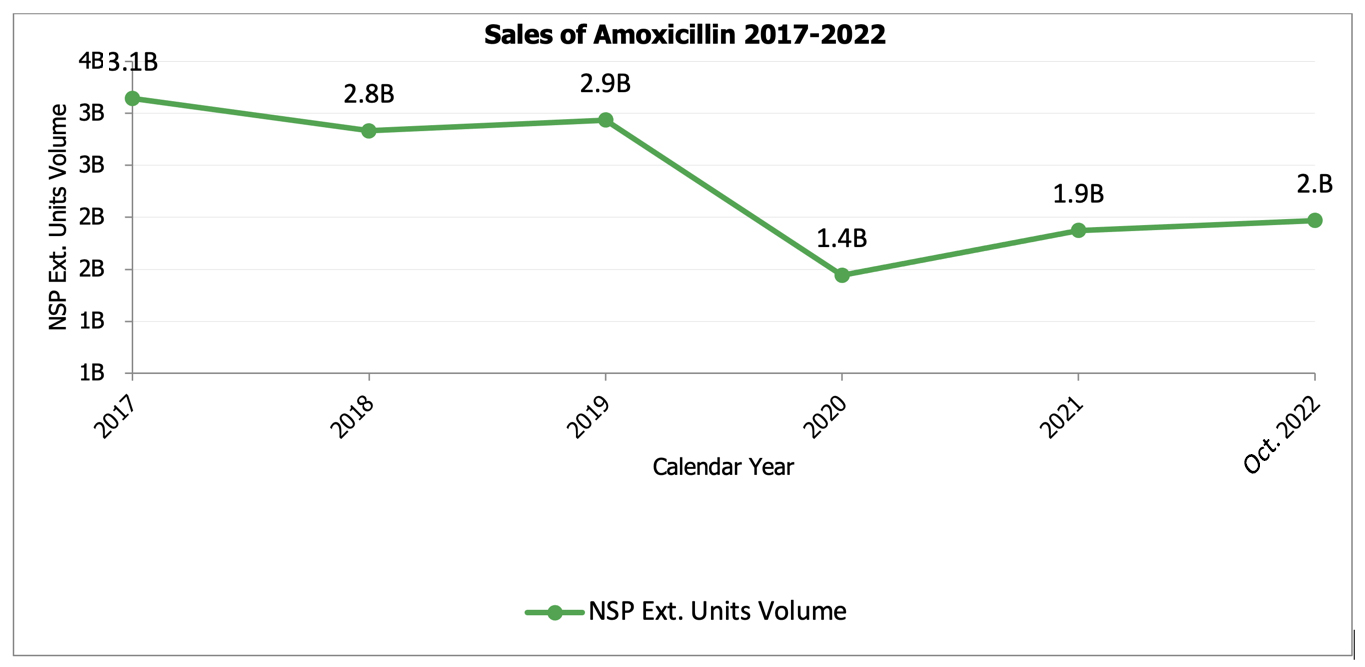
Over the last two years, demand for amoxicillin pediatric suspension declined by more than 50 percent. Approximately 1.4 billion doses of amoxicillin pediatric suspension were sold in 2020 and then 1.9 billion doses in 2021. However, as of October 2022, generic manufacturers have already produced more than 2 billion doses of amoxicillin pediatric suspension – more than previous years.
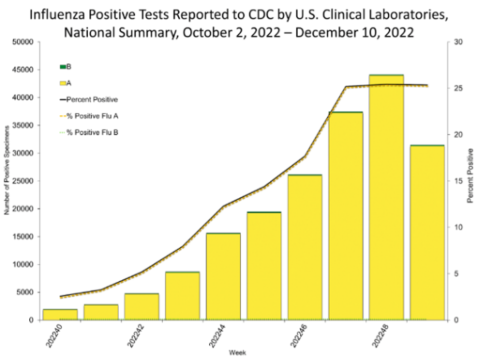
Throughout the fall, the number of patients with influenza has rapidly increased. The Centers for Disease Control reported less than 5,000 cases in early October and then by early December, the number of cases reached 45,000 in the first week.
In addition, children are now experiencing RSV at unprecedented rates and some health care experts are seeing the signs of another COVID surge in parts of the country.
Physicians, pharmacies, distributors, and other purchasers were understandably unable to predict the increases in influenza, RSV and COVID cases – nor could they forecast the impact of all three occurring at the same time.
The same is true for generic manufacturers.
Six generic manufacturers currently produce amoxicillin pediatric suspension and sell the medicine at list prices ranging from $4 to $7 for a course of treatment. Since 2016, no manufacturers have exited the market. Moreover, AAM members report there is sufficient supply and access to active pharmaceutical ingredients (APIs) and key starting materials (KSMs) used in the production of amoxicillin.
While some claim the inability of patients to fill amoxicillin prescriptions is due to a broken generic drug market, this rhetoric is not supported by the facts. As former FDA Commissioner Scott Gottlieb recently explained on CBS’s Face the Nation
on December 18: Demand went up this year, they anticipated some increase in demand, but not as much as we’re seeing and not this early in the season…So it’s not any kind of disruption in supply. This isn’t like what we had with baby formula where manufacturers have been taken out of the market.
Generic manufacturers do face an array of market forces that jeopardize the long-term sustainability of low-cost medicines. The consolidation of purchasers contributes to significant pricing pressure and a multi-year deflationary cycle for generics that threatens continued patient access to the lowest cost medicines. Combined with rebate practices that too often favor high-cost, high-priced, brand-name drugs over lower-cost generic competition, generic manufacturers experience a challenging business environment, and patients often pay too much for prescriptions when more affordable alternatives are available.
Generic manufacturers are doing their part to increase the supply of amoxicillin pediatric suspension and other low-cost antibiotic treatments in the face of unprecedented demand due to the tripledemic this winter. While no patient should ever go without access to an FDA-approved, physician prescribed medicine, it is important to properly diagnosis what is causing limits to supply before rushing to judgement. Action is needed to address the long-term sustainability of generic manufacturers, enhance the U.S. pharmaceutical supply chain, and ensure patients are able to access generic medicines when they need them. We stand ready to work with policymakers on how best to do so.
By Craig Burton, Senior Vice President, Policy and Strategic Alliances
Executive Director, Biosimilars Council
Published on December 21, 2022