COVID-19 Response: Roby Epting at Sandoz

Roby Epting is a manager of second-shift operations at Sandoz in Wilson, North Carolina. AAM is recognizing dedicated people like Roby and his peers across the generics and biosimilars industry who show up each day for U.S. patients. “We are just excited to be able to contribute in any kind of way.” Learn more at: […]
COVID-19 Response: Anthony Maffia at Sandoz

Anthony Maffia works in New Jersey at Sandoz as Vice President, Head of Regulatory Affairs, North America. AAM is recognizing dedicated people like Anthony and his peers across the generic & biosimilar industry who show up each day for US patients. The industry is “finding new and creative and rapid ways to get medication into […]
Introduction to the Generic Drug Supply Chain
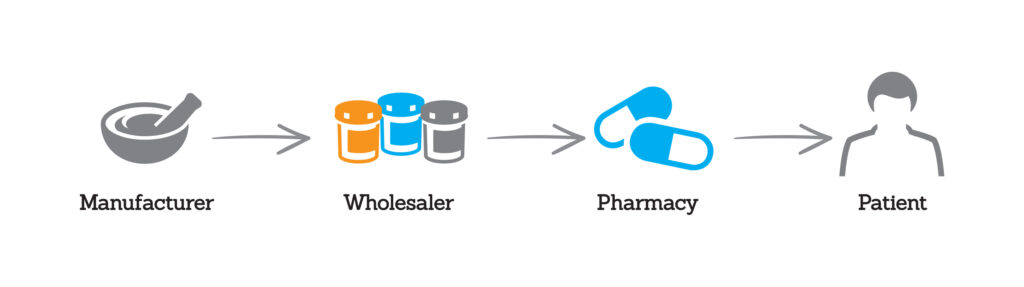
It is important that policymakers consider the differences inherent in the branded and generic prescription drug markets when considering public policy changes concerning drug pricing. The differences between the respective markets may require solutions tailored to the specific challenges, rather than a one-size-fits-all solution. In doing so, policymakers should carefully consider how to ensure the […]
Generic Drugs – Facing Threats to Sustainable Competition & Supply

AAM presentation delivered at the Federal Trade Commission Workshop, November 8, 2017. The future of a sustainable competitive supply of life-saving affordable generic medicines is under threat – the result of a combination of anticompetitive behavior, market consolidation, and policy missteps. We encourage the agencies, Congress, and the public to be vigilant in assessing the […]
Open configuration options Generics and Biosimilars Industry Supply Chain & Response to COVID-19
This presentation includes: AAM Member Action: AAM members are working 24/7 to assure access and donating medicines and masks in response to the urgent needs created by the pandemic. Supply Chain Visuals: How medicines actually get to patients is of increasing interest. These simplified schematics show the step-by-step journey of a generic or biosimilar drug from […]
COVID-19: This Week’s Initiatives From the Generic and Biosimilars Industry

Third in a series, view first and second. As the COVID-19 pandemic progresses, Americans are doing their part, working from home, studying online and practicing social distancing. Developers and manufacturers of generic and biosimilar medicines are doing their part as well, working around the clock to ensure America’s patients have access to the medicines they need. […]
Dispensary of Hope: The Industry’s Nonprofit Supply-Chain Partner

The expo earlier this month at the second annual GRx+Biosims conference gave attendees the opportunity for dialogue with regulators, vendors and other stakeholders in the generic and biosimilars industry. Each participant offered a unique perspective, but only one group that appeared could claim to fill 1.1 million prescriptions during more than 487,000 encounters last year. […]
AAM Testimony on the Generic Global Supply Chain—Five Takeaways

On Wednesday, David Gaugh, R.Ph., AAM’s Senior Vice President, Sciences & Regulatory Affairs, provided testimony to the House Energy and Commerce Health Subcommittee hearing, “Safeguarding Pharmaceutical Supply Chains in a Global Economy.” Here are five important takeaways from his remarks. Patient safety is the number one priority for AAM and its member companies. Generics […]
House Subcommittee Examines the Drug Supply Chain

AAM President and CEO Chip Davis testified before the U.S. House of Representatives Energy and Commerce Health Subcommittee December 13 at its hearing, “Examining the Drug Supply Chain.” The oldest standing legislative committee in the U.S. House of Representatives, this body is vested with the broadest jurisdiction of any congressional authorizing committee. In his testimony, […]
The Generic Drug Supply Chain
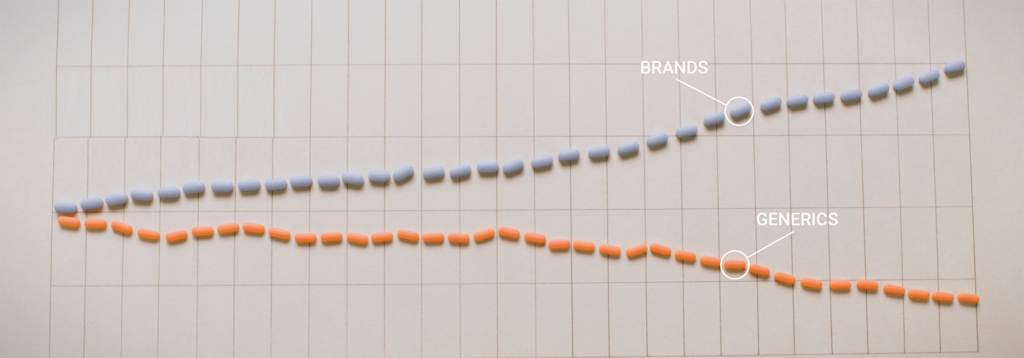
With 89 percent of all prescriptions dispensed in the U.S. last year filled with a generic drug — but those scripts accounting for only 26 percent of total drug costs — generic medicines play a crucial role in the U.S. health care system. To keep lifesaving generics available, it’s important that policymakers understand the supply […]
AAM Calls for FTC Action to Ensure U.S. Supply of Generic Drugs for Patients

Association for Accessible Medicines files comments with FTC addressing buyer consolidation and anti-competitive tactics “Today AAM is calling on the FTC and other federal agencies to take action against anti-competitive practices and consolidation of large-scale buying groups that are threatening the future supply of affordable generic and biosimilar medicines in the U.S. FDA-approved generic medicines […]
