Generics and biosimilars—cornerstones of the U.S. healthcare system—have provided a reliable safety net for millions of Americans, ensuring access to vital therapies at the lowest possible cost. Yet, a perfect storm of federal regulatory hurdles, patent abuses, and flawed policies threatens the very foundation of this system.
Generics and biosimilars are essential to the U.S. healthcare system and America’s patients. These medicines account for 90% of prescriptions dispensed in the U.S. but only 12% of total drug spending. Over the past decade, their widespread adoption has saved the healthcare system an astounding $3.4 trillion. This success is not just an economic achievement; it is a lifeline for patients who depend on affordable medicines to manage chronic conditions, treat serious illnesses, and maintain a quality of life.
But this success story is in jeopardy. The overall value of generic sales in the U.S. has declined by $6.4 billion since 2019, despite increased utilization and new product launches.
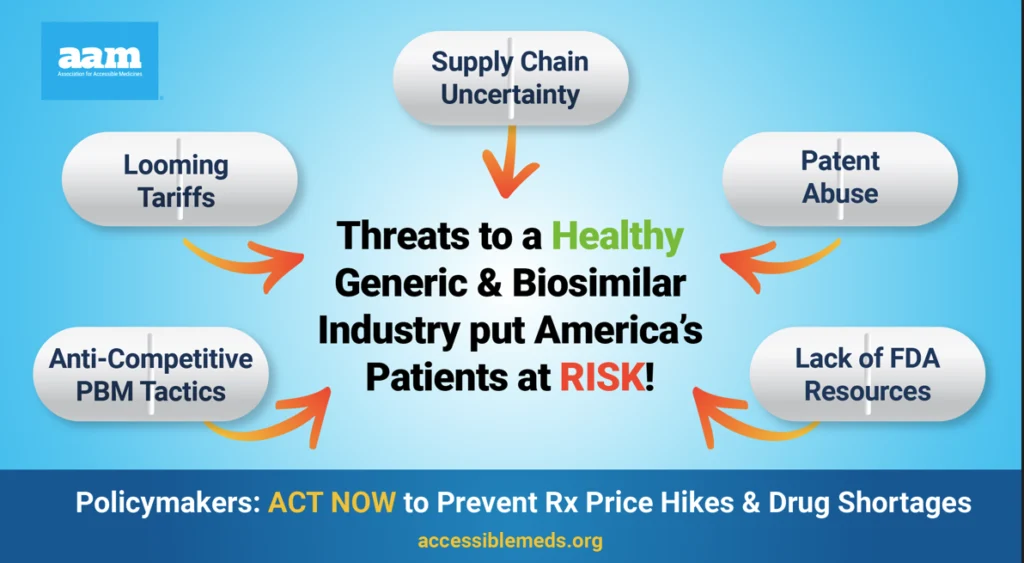

Regulatory Barriers: Outdated and unnecessary FDA requirements delay the development and approval of lower-cost generics and biosimilars.
Patent Abuse: Brand-name drug manufacturers exploit the patent system to extend monopolies well beyond the original patent term, creating “patent thickets” that block generic and biosimilar competition.
Flawed Policies:
Overall Sustainability: Due to a lack of adequate reimbursement, generic medicines are increasingly at risk of shortages. Without systemic reforms to stabilize and incentivize the generic drug supply chain, patients could face dangerous interruptions in their treatment.
Policymakers must act decisively to address these systemic threats. This requires a comprehensive approach:
In 2023, generic and biosimilar prescription medicines saved $445 billion for the U.S. healthcare system overall, with more than $3 trillion saved in the past 10 years.
