Increasing the domestic production of essential medicines is one of the few issues that Republicans and Democrats both support. Since the onset of the COVID pandemic, more than 50 bills have been introduced in Congress, many bipartisan, to encourage and support this production. Both the Trump and Biden administrations also presented strategies to enhance the resiliency of the supply chain through the onshoring and nearshoring of essential medicine manufacturing.
Since taking office, the Biden administration has published several key reports1 that identify strengths and vulnerabilities in the U.S. pharmaceutical supply chain and propose a range of incentives and programs to expand this production. Many of these proposals align well with the Association for Accessible Medicines’ Blueprint for Enhancing the Security of the U.S. Pharmaceutical Supply Chain. And, if implemented and properly funded, these proposals could have a real impact on incentivizing greater production of generic medicines in the United States.
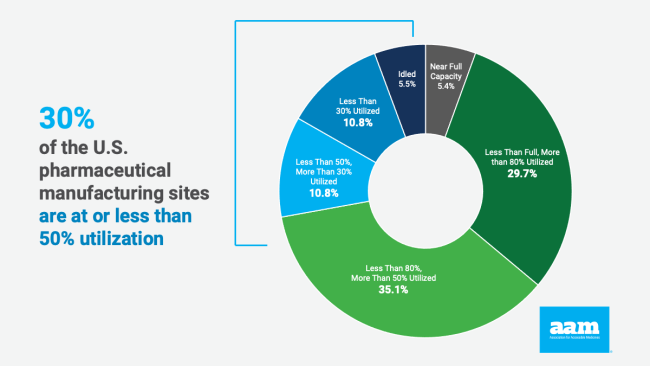
Based on a report published last fall by Professor Anthony Sardella from the Washington University, the best and most efficient means of expanding this production is relying on the manufacturing sites that already exist in the United States. According to Professor Sardella’ s research, 49% of the generic pharmaceutical manufacturing capacity in the United States is currently idle. Of the 37 manufacturing sites surveyed, only two were at full capacity, while 30% were at or less than 50% capacity.
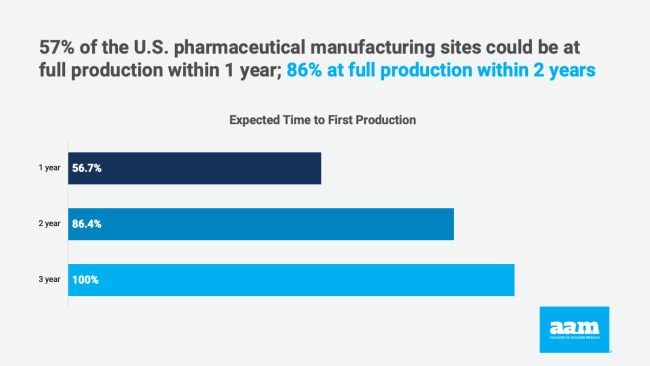
Launching a new facility can cost hundreds of millions of dollars and take years to build. However, this research demonstrates that manufacturers with excess capacity can repurpose their production lines to make medicines on the FDA’s Essential Medicines List relatively quickly. The companies surveyed indicated that 57% of the sites could be at full production within one year and 86% within two years.
Leveraging the excess capacity of generic manufacturing sites already established in the United States is the quickest and least expensive way to expand domestic production of essential medicines. And to support this approach, it will be critical for the federal government to be a partner in making this sustainable over the long run. For example, long-term price and volume contracts would incentivize manufacturers to invest in and expand production. Tax incentives, grants and other tools would enable U.S.-manufactured generic medicines to predictably compete against less expensive imports. AAM’s Blueprint provides further details on the incentives needed to achieve this shared goal.
As Professor Sardella’ s research demonstrates, generic manufacturers are ready to make more essential medicines in the United States. Now is the time to leverage the bipartisan support for expanding domestic production and put in place the policies that can help make that happen.

By Jonathan Kimball, AAM Vice President, Trade and International Affairs
Published on February 9, 2023
References
- Building Resilient Supply Chains, Revitalizing American Manufacturing, and Fostering Broad-based Growth: 100-Day Reviews under Executive Order 14017, The White House (June 2021); Public Health Supply Chain and Industrial Base One-Year Report, ASPR, Department of Health and Human Services (February 2022); Essential Medicines Supply Chain and Manufacturing: Resilience Assessment, The Advanced Regenerative Manufacturing Institute (on behalf of HHS/ASPR); May 2022.

